is a non-invasive test for the screening of colorectal cancer and precancerous lesions.
About the test

It can detect abnormalities which, if confirmed by a colonoscopy, allow for the early diagnosis of colorectal cancer. In fact, if discovered early on, colorectal cancer can be treated more effectively and less invasively. An early evaluation often allows to identify and eliminate precancerous lesions (polyps), avoiding the onset of the tumor.
Indications
In general, for subjects presenting a risk similar to that of the general population , it is advisable to adhere to a screening protocol after the age of 50.
For those at risk for family history, it is best to start 5-10 years before the age of the family member (first degree) being diagnosed with colon cancer.
Finally, for those who have a high risk, i.e. cases of proven genetic predisposition (about 5-10%), screening must be started very early.

Why perform a screening?
It is important to adhere to a screening program because, if discovered at an early stage, colorectal cancer can be treated more effectively and less invasively . This precocity often allows the identification and elimination of precancerous lesions (intestinal polyps), avoiding the onset of the tumor.
RISK FACTORS
Risk factors are quite general and cross-cutting in the general population, both men and women.
NUTRITIONAL FACTORS
A diet low in fiber, rich in fats and proteins of animal origin is associated with an increase in intestinal tumors. Obesity, overweight and an unhealthy lifestyle are additional risk factors.
NON HEREDITARY FACTORS
The risk of getting colorectal cancer is determined by factors such as age, smoking, sedentary lifestyle, chronic inflammatory bowel disease, a history of colon polyps or previous colorectal cancer.
GENETIC FACTORS
It is possible to inherit the predisposition to get colorectal cancer (about 10% are hereditary forms). Through specific genetic tests (MyCheck) it is possible to verify a possible genetic predisposition.
STATISTICAL INFORMATION ON COLON RECTUM CANCER
- It accounts for approximately 10% of all cancers diagnosed in the world
- In Italy it is the second most frequently diagnosed cancer
- It is especially common in the 50 – 70 age group
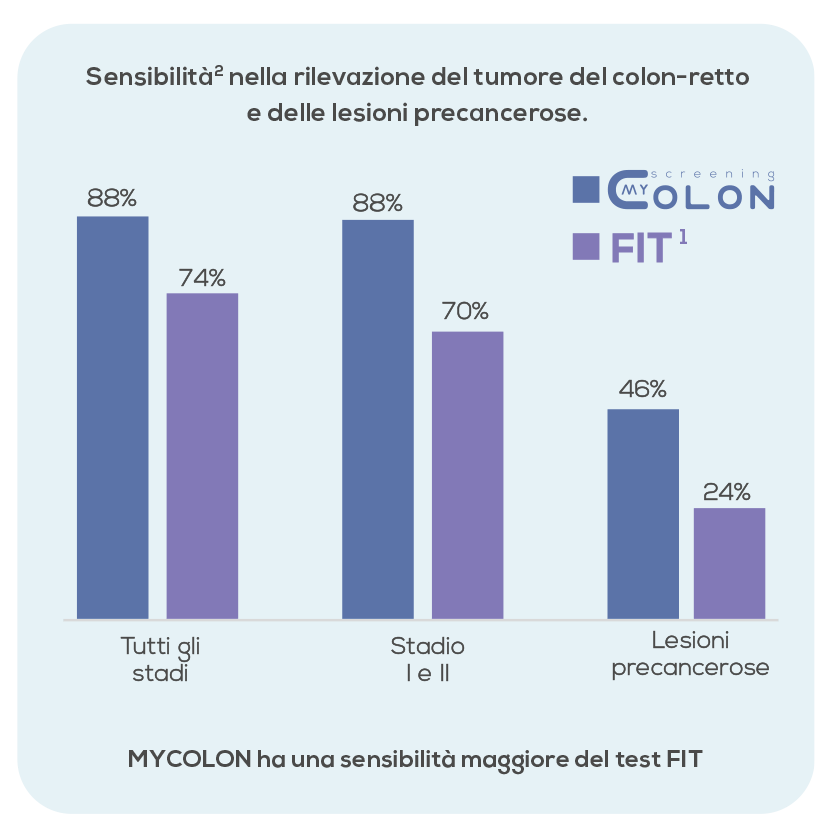

Test Advantages

- Does not require preparation (diet, laxatives)
- A stool sample is sufficient
- Analyzes 2 markers present in DNA
- Detects the presence of occult blood in the stool
- Has a sensitivity that can reach 96%
- Has a specificity that can reach 87%
- Has a sensitivity that can reach 52% for advanced precancerous lesions

How the test is performed
DNA is extracted from a stool sample and subjected to a specific analysis (Multiplex Fluorescent PCR) to detect possible methylation abnormalities. The test is performed on stool, which can be conveniently collected at home. The innovative approach of MyColon 3.0 consists of combining the test for the detection of occult blood in stool (FOBT), already included in most regional screening programs, with a more sophisticated DNA analysis aimed at identifying possible methylation defects in 2 genes (ADHFE1 – OPLAH), markers of CRC. This combination increases the accuracy of the final screening result, especially in the case of precancerous lesions.
Request the test
To request MyColon 3.0 or to obtain further information on the test, fill out the form below, or call +971 (0)4 375 7220 . Our biologists will respond to your requests without any commitment on your part.
(*) Required fields