Unlike the “targeted sequencing” methods used for some older generation NIPT tests, or other economic solutions without adequate clinical validation, the UltraNIPT methodology, through the analysis of the whole genome and advanced bioinformatics analyses, provides highly accurate results and a wider range of detectable abnormalities: trisomies, sex chromosome aneuploidies and deletion/duplication syndromes affecting the entire chromosomal makeup.
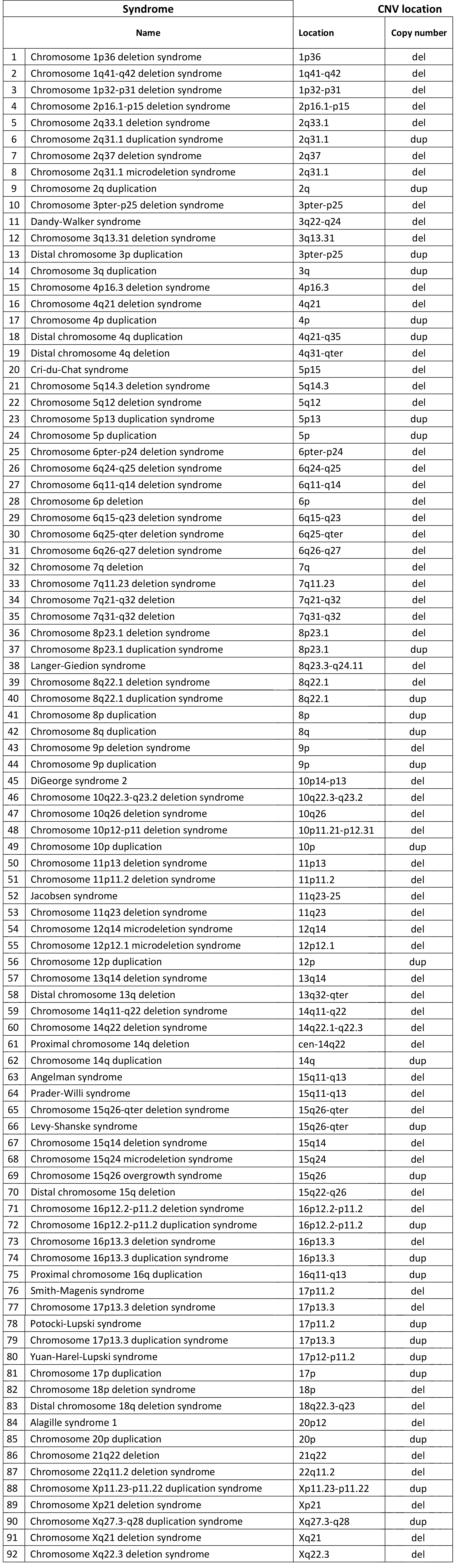
Chromosomal anomalies analyzed with the ULTRANIPT 2.0 test

Indications
The UltraNIPT 2.0 test is recommended in case of:
- Single pregnancies
- Single pregnancies in which invasive testing is not intended
- Single pregnancies in which invasive prenatal diagnosis is indicated
- Single pregnancies from assisted insemination (also from egg donation)
Advantages
- It is based on a simple maternal blood sample
- It can be performed starting from the 10th week of gestation
- Provides compensation in the event of failure to detect (*)
- It provides a reimbursement of expenses in case of an anomaly for any in-depth diagnostic and/or genetic counseling (*)
- The report includes the direct link to the OMIM (Online Mendelian Inheritance in Man) page of the detected syndromes
Reliability
- The only test in the world capable of detecting 118 chromosomal anomalies with relative clinical interpretation
- A clinical study of over 146,958 women makes it by far the most validated among the other tests on fetal DNA
- More than 5 million tests performed worldwide
- Sensitivity greater than 99% for trisomies 21, 18 and 13
- Sensitivity greater than 90% for detecting deletions and duplications, even sub-microscopic (up to 3Mb)
- The WIDE option can detect sub-microscopic anomalies (up to 1Mb) affecting all chromosomes
Quality assurance
The guarantee of reliability, coming from the widest case history in the world (more than 5 million tests performed), has made it possible for each pregnant woman who requests it, access to a free insurance policy (*). In case of failure to detect some aneuploidies, it is possible to receive compensation or, in the case of in-depth diagnostic and/or genetic counseling, a reimbursement of expenses.
* There are some restrictions. For more information, you can contact the Bioscience Institute before undergoing the test.
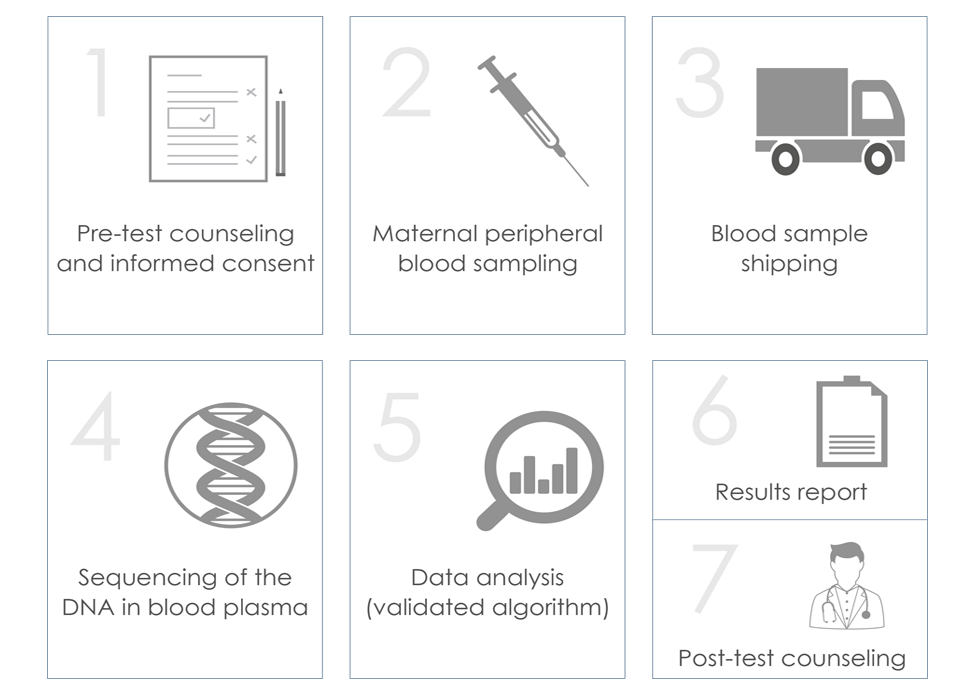
Procedure
The ULTRANIPT 2.0 test is performed on a maternal peripheral blood sample of approximately 8 ml, taken between the 10th and 24th week of gestation with a CE-IVD certified test tube. The blood taken is transported inside an isothermal kit for category B biological material, in compliance with the UN3373 standard.
The test involves the extraction of DNA from the plasma, and sequencing and analysis using specific bioinformatic calculation algorithms that allow the detection of 118 genetic syndromes.
Results management
Results are available in approximately 8 working days. It is important to point out that durations may vary: the analysis involves a series of rigorous quality controls aimed at guaranteeing the reliability of the result; among these is the verification of the quantity of fetal DNA present in the blood sample, which varies from one pregnant woman to the other. In some cases, due to the low quantity of fetal DNA, it is necessary to repeat the analysis and/or blood sampling. If it is necessary to repeat the test due to the low concentration of fetal DNA in the maternal blood, the repeat will be free of charge.
Request UltraNIPT 2.0
The prenatal test with a more extensive and targeted level of screening.
Contact Bioscience Institute to find the Center closest to you where you may book the ULTRANIPT 2.0, or fill out the request form to be contacted without obligation by one of our trusted biologists.
(*) Required fields
References
• Zhang H, et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146.958 pregnancies. Ultrasound Obstet Gynecol.2015 Jan 19.
• Chen S, et al. A method for noninvasive detection of large fetal deletions/duplications by low coverage massively parallel sequencing. Prenat Diagn.2013
• Liu et al. Performance evaluation of NIPT in detection of chromosomal copy number variants using low coverage whole genome sequencing of plasma DNA. Plos One, 2016
• Pan X, et al. Noninvasive fetal sex determination by maternal plasma sequencing and application X-linked disorder counseling. J.Matern Fetal Neonatal Med. 2014 Dec.
• Jiang et al. Noninvasive fetal Trisomy test: an advanced noninvasive prenatal diagnosis methodology for fetal and sex chromosomal aneuploidies. BMC Medical Genomics, 2012.


