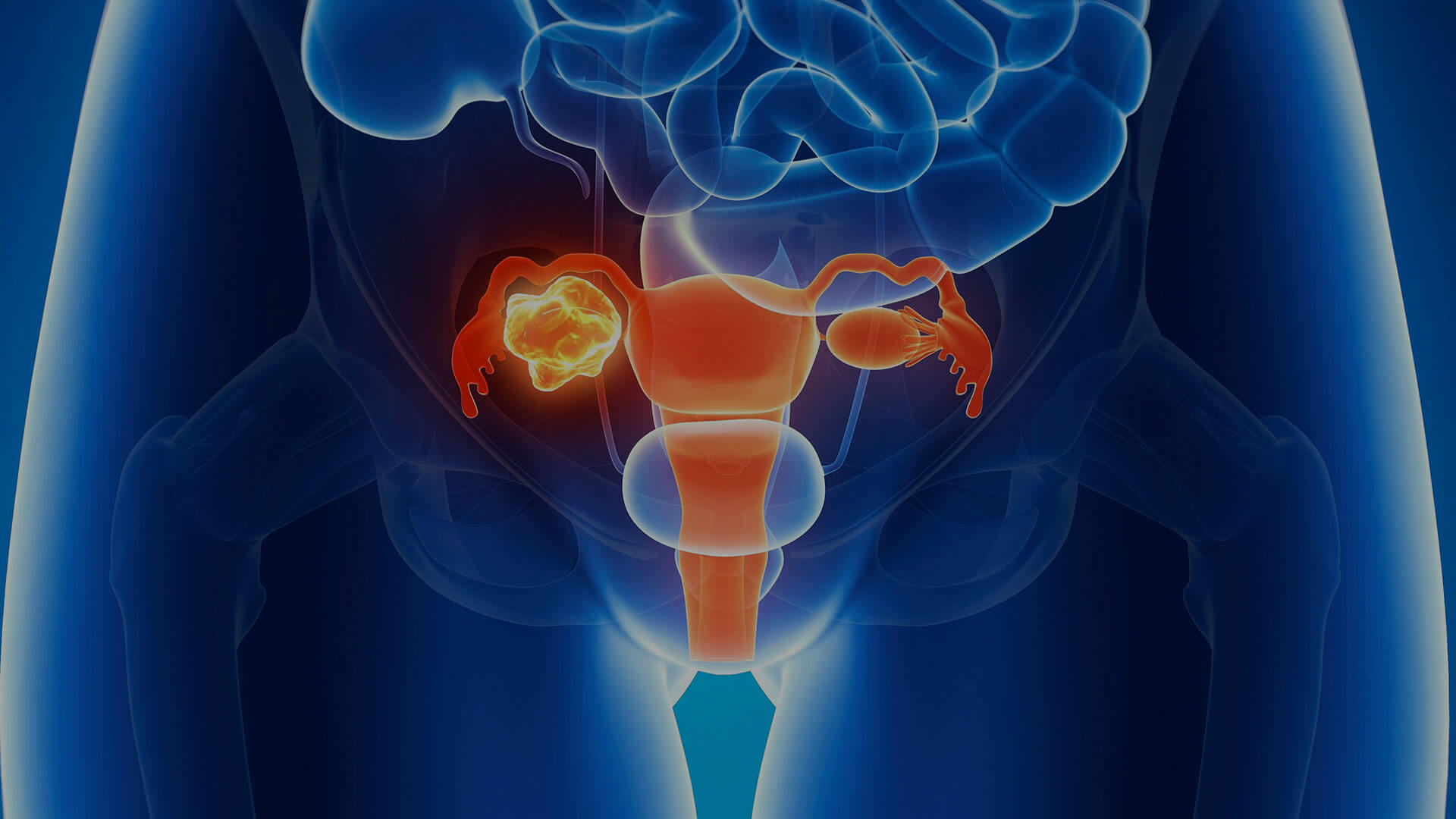
HELIXGYN is the elective monitoring program for those who have inherited the mutation in the BRCA 1 and BRCA 2 genes and therefore express a high risk of developing breast or ovarian cancer. HELIXGYN is also an interception weapon for women who are taking (or have taken) hormone replacement therapy (HRT) or hormonal contraceptives, or who have undergone ovarian stimulation.

Breast and ovarian cancer interception
Indications
The HELIXGYN prevention program is recommended for all women:
-
- who are familiar with the mutation in the BRCA 1 and BRCA 2 genes, and therefore are at greater risk of developing breast or ovarian cancer;
- who are taking (or have taken) hormone replacement therapy (HRT) or hormonal contraceptives;
- who have undergone ovarian stimulation.
The risks of hormone replacement therapies (HRT), contraceptives or ovarian stimulation.
Hormone replacement therapies

The relationship between hormone replacement therapies in menopause and the risk of developing some forms of cancer has been a rather debated topic for decades. The use of hormone replacement therapy to cope with menopause increases the risk, in proportion to the duration of treatment, of developing breast cancer, and makes diagnosis difficult. Taking estrogen alone increases the risk of endometrial hyperplasia, a condition that may precede the development of endometrial cancer.
Hormonal stimulations

It is thought that prolonged hormonal stimulation can expose some sensitive tissues of the breast, uterus, cervix, and ovaries to the development of tumor formations. Although there is no evidence of the link between infertility treatment and cancer, we do know that hormones can accelerate the growth of cancer cells in some tissues. For this reason, we recommend starting the HELIXGYN program in parallel with the infertility treatments based on hormonal stimulation; in this way it is possible to monitor the stability of the genes indirectly involved in the treatment.
Hormonal contraceptive therapies

Hormonal contraceptive therapies contain estrogen and/or progesterone which can stimulate the growth of endocrine responsive tumors (i.e., which grown on hormonal stimulation). The Oxford Family Planning Association Contraceptive Study, which involved around 17,000 women in the UK, analyzed the risk as above average. HELIXGYN solves doubts concerning the safety of contraceptive hormonal treatments by monitoring the mutations that indicate genetic instability (and therefore the risk).
What the test evaluates
HELIXGYN evaluates the genetic stability and therefore the safety conditions in which hormonal therapies are carried out by monitoring the frequencies of somatic mutations in the genome, with special attention for the alterations more sensitive to that type of stimulation. Before starting any hormonal therapy, it would therefore be advisable to start a monitoring program, with annual repetition, of the mutational profile.
An innovative model
The assessment of the risk of solid tumors has always been based on the study of family history.
With the HELIXAFE program it is possible to evaluate objective parameters, such as somatic or acquired mutations, which can be analyzed using the most modern techniques of liquid biopsy and sequencing (Next Generation Sequencing). As a result of NGS, it is possible to monitor these mutation frequencies in the patient and then analyze the genomic instability through the algorithm patented by Bioscience Institute.
How the test is performed
The Bioscience Institute offers pre-test advice and provides all useful information about the HELIXAFE prevention program and the execution of the HELIXGYN test.
The test involves a simple blood sample (10-20 cc of blood) which can be performed at one of our reference centers or through your doctor.
The Bioscience Institute laboratories extract the DNA present in the sample and sequence it using the advanced Next Generation Sequencing (NGS) techniques. The subsequent bioinformatics analysis makes it possible to determine the presence of any mutations in the genes under examination.
Test results are available in approximately 30 days

Request HELIXGYN
Request the HELIXGYN test to find out your level of genetic stability.
Email info@bioinst.com or fill out the form below to be contacted by one of our experts.
(*) Required fields