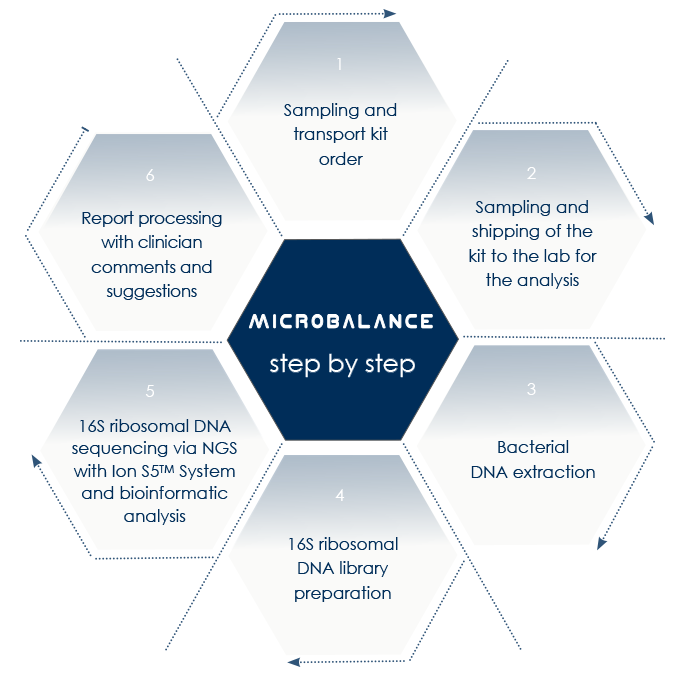
MICROBalance by Bioscience Institute is a platform for the analysis of microbioma (the genome of the microbiota) aimed at identify microbiota alterations and correct them by means of lifestyle-based approaches and, if needed, food supplements.

MICROBIOMA AND MICROBIOTA
Human organism is not a sterile environment: microbes are present both on its surface and inside it, where they contribute to its health status.
This community of microbes (bacteria, fungi, protozoan, and viruses) is called microbiota and comprises 100 billion billion bacteria.
The genome of all of these microorganisms is called microbioma.
Gut microbiota features
Human microbiota inhabits different body districts: oral cavity, stomach, small intestine, and colon; ear, nose and lung; urinary tract; vagina; and skin. The term “gut microbiota” refers to microbes living in the intestines – a community 10 fold more abundant than human body cells and whose genome (the so called “gut microbioma”) encodes a number of genes 100 fold greater than genes in human genome.
This community, also known as “gut flora”, is more abundant in the colon, where there are 100 billion bacteria each gram of intestinal content.

MICROBIOTA FUNCTIONS
Microbiota acts as a shield against pathogens that could invade the skin, the oral cavity, the digestive tract, and the vagina. It is fundamental for immune system and immune tolerance development. Moreover, it plays a fundamental role in the digestion and the absorption of food components (in particular, minerals and fiber), in the synthesis of some vitamins and amino acids, in the production of molecules that regulate immune and metabolic responses, in the detoxification of potentially dangerous compounds (such as carcinogenic molecules), and in the inactivation of some medicines. However, in some circumstances pathogens can proliferate, promoting health problems also outside the digestive system, such as urinary tract infections (UTIs) and bacterial vaginosis.
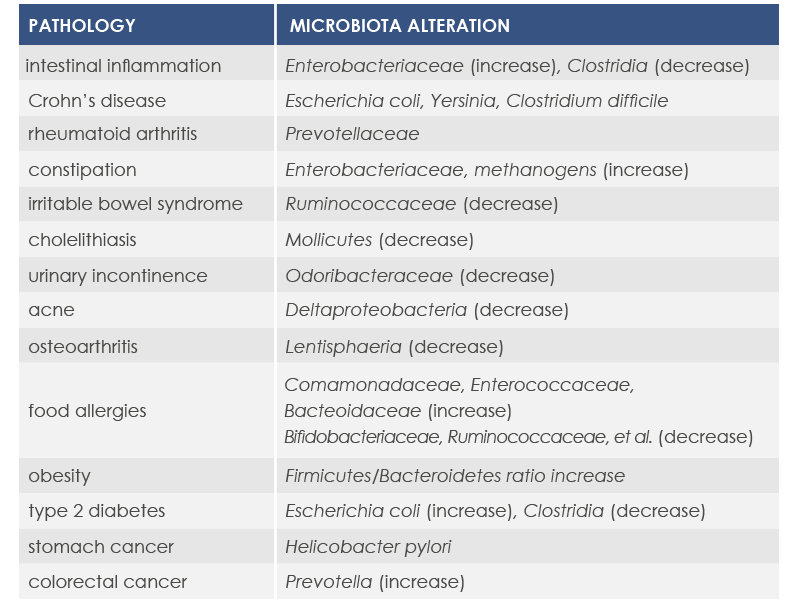
These and other dysbiosis (microbiota alterations) are associated with both gastrointestinal acute inflammations and other inflammation-associated conditions, such as the so called inflammaging, that is the inflammation associated to aging.
GUT AND VAGINAL MICROBIOTA, UTLS AND VAGINAL INFECTION
Dysbioses of urinary trait microbiota are often characterized by a significative increase in several strains of Escherichia coli, often harboring multidrug resistance. Gut microbiota is a reservoir of such microbes.
In women vaginal microbiota could be involved too. In fact, the first step in UTIs pathogenesis is vaginal introitus or urethra colonization. From there, pathogens can move to the bladder and, sometimes, the kidney.

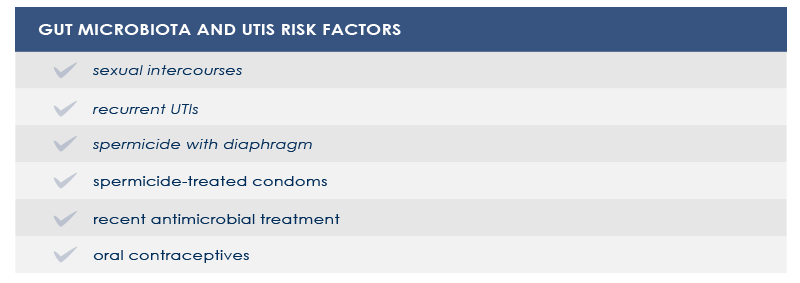
In other cases, vaginal microbiota is a gut-independent pathogen reservoir. In particular, the loss of protective Lactobacillus species increases the risk of both UTIs and bacterial vaginosis or infections by pathogens such as Neisseria gonorrhaeae. Among vaginal microbiota altering factors are antimicrobial treatment and spermicide use.
Strategies aimed at counteract gut and vaginal microbiota alterations could help decrease the risk of UTIs and vaginal microbiota-related conditions.
GUT AND VAGINAL MICROBIOTA, PREGNANCY AND BREASTFEEDING
The link between microbiota and human health develops since the very first moments of life. That is why it is important to take care of gut flora wellbeing during pregnancy and breastfeeding.
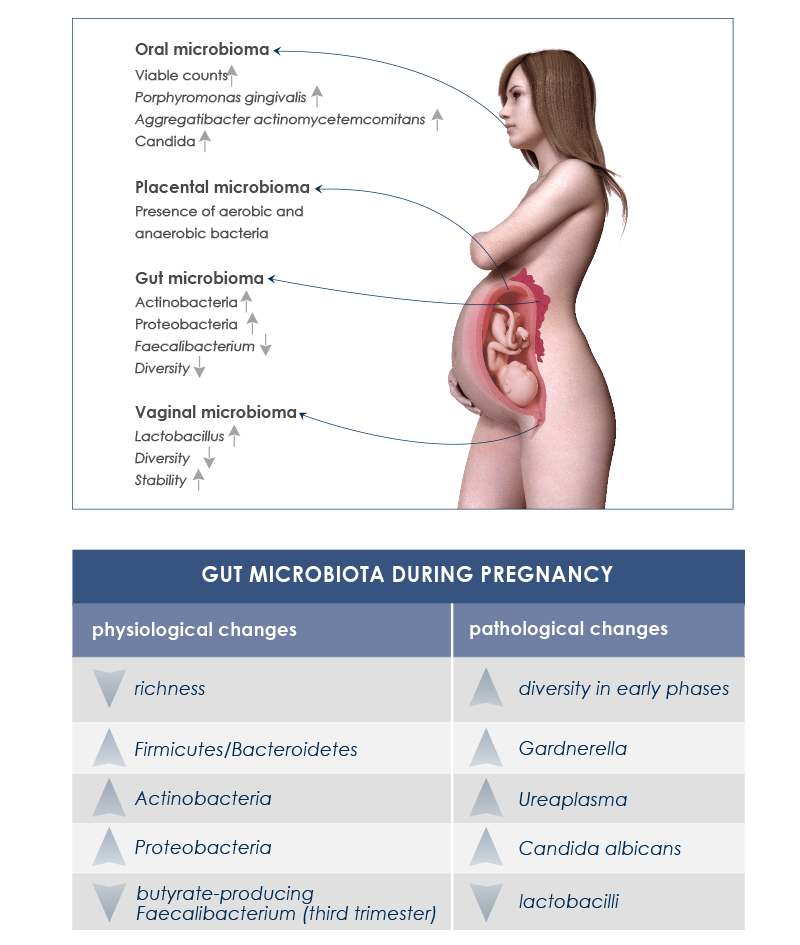
Endocrine, metabolic and immune changes taking place during pregnancy are associated with significative gut microbiota variations. It is important to distinguish between healthy changes – needed for the physiologic maternal weight increase and for fetus nutrition – from undesirable ones that could lead to health complications or compromise good baby gut microbiota development.
Moreover, during pregnancy vaginal and oral cavity-associated microbiota change too. Vaginal microbiota helps avoid infections. During pregnancy its diversity decreases, whereas its stability increases; Lactobacillus species increase too, whereas pH decreases. Instead, after delivery vaginal microbiota becomes more similar to gut microbiota, some Lactobacillus species are lost, diversity increases, and vaginosis-associated bacteria are more abundant. In the oral cavity, periodontal disease-associated pathogens and Candida levels increase.
The presence of gut microbes in the amniotic fluid of women with preterm rupture of membranes suggests that gut microbiota plays a role in intrauterine infections too.
Moreover, there is another good reason to maintain a healthy microbiota during pregnancy: the gut of children born by vaginal delivery is first colonized by bacteria in the vagina and the gut of their mother.
It has been hypothesized that in case of cesarean section the absence of this transmission could have long-term consequences on child health, such as obesity, asthma, and celiac disease development. Moreover, Bacteroides, which are most abundant after vaginal delivery, influence immune system maturation.
Breastfeeding plays a fundamental role in baby’s gut microbiota development too. During the first year of life microbial composition of breast milk changes. Since bacteria in breast milk come from the gut, taking care of gut microbiota during breastfeeding is fundamental too.

GUT MICROBIOTA AND IMMUNE SYSTEM
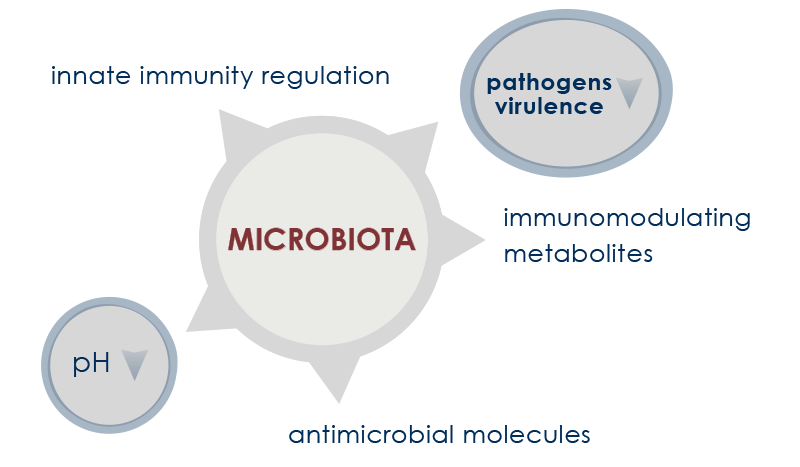
Gut microbiota is deeply involved in immune system induction, education, and functioning. The interplay between microbes and immunity starts developing during breastfeeding and reinforces for life the so called “barrier immunity” (that is, the mechanisms that minimize the contact between microorganisms and intestinal cells).
The presence of a healthy gut flora guarantees good immunity functioning both in physiological conditions and in the presence of inflammatory diseases. Moreover, it protects tissues from inflammation-associated damage.
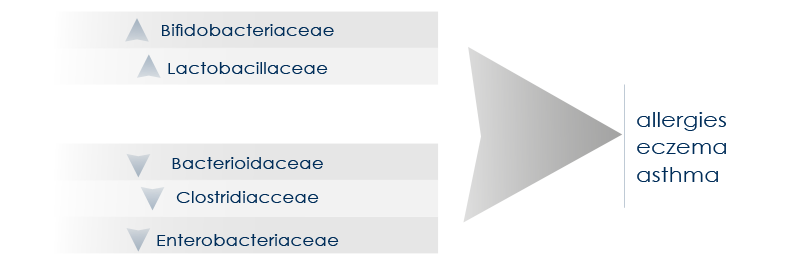
A microbiota characterized by good features (such as a greater diversity) is associated with the reduction of the risk of pathologies such as asthma and allergies, whereas gut dysbiosis can be associated with asthma, allergies, and eczema.
Gut microbiota alterations can influence immune responses both at the local level and in organs distant from the intestines. For example, “good” bacteria reduction associated with antibiotic treatment can decrease the response to influenza virus infection in the nose. The control operated by gut microbiota on systemic immunity exerts profounds consequences on possible therapies. For example, the effect on microbiota of radiotherapy associated to some immunotherapy protocols promotes bone marrow transplantation good results. On the contrary, gut microbiota changes induced by antibiotics can compromise the response to immunotherapy.
GUT MICROBIOTA, MOOD, AND BEHAVIOR
Just as brain influences gut functioning, so too mood and behavior depend on gut microbiota. This relationship rests on immune signals traveling from the gut to the brain, on the interaction between microbiota and gut wall, and on the contact between microbes and nerve terminals in the intestine.
The alteration of inflammatory responses caused by dysbiosis promotes a status of chronic inflammation that is associated with mood and behavior changes, greater stress reactivity, and greater stress-associated diseases incidence.
Moreover, gut microbiota can influence eating habits. In fact, nerve and chemical signals from the gut are elaborated by central nervous system to regulate appetite and food intake.
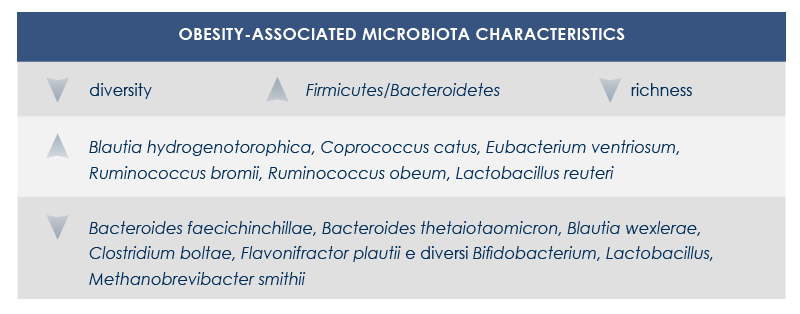
Gut microbiota and obesity
Obesity and obesity-related diseases are associated with gut microbiota changes. This relationship depends at least in part on energy intake regulation by microbiota. In fact, by fermenting fiber microbes extract additional energy from food. Moreover, fiber fermentation leads to the production of short-chain fatty acids that promote lipogenesis, triglycerides stockage, and adipocyte differentiation; at the same time, it inhibits lipolysis.
Also the better digestion of complex carbohydrates seems to increase energy extraction from food. Finally, obesity-associated gut microbiota reduces fatty acids oxidation and modifies gene expression in colon cells; this leads to the alteration of triglycerides metabolism and promotes fatty acids accumulation.
Obesity-related diseases are influenced by shifts in some bacteria levels (for example, the decrease of clostridia and the increase of lactobacilli associated with insulin resistance), the decrease of microbiota richness (associated with both insulin resistance and dyslipidemia), changes in hormones and other molecules produced by endocrine cells in the intestine, alteration of gut-brain communications and of signals to the adipose tissue. Finally, dysbiosis can increase gut permeability, letting microbial molecules such as lipopolysaccharide (a bacterial toxin that can lead to systemic inflammation) cross gut barrier.
MICROBIOTA AND INFLAMMATION
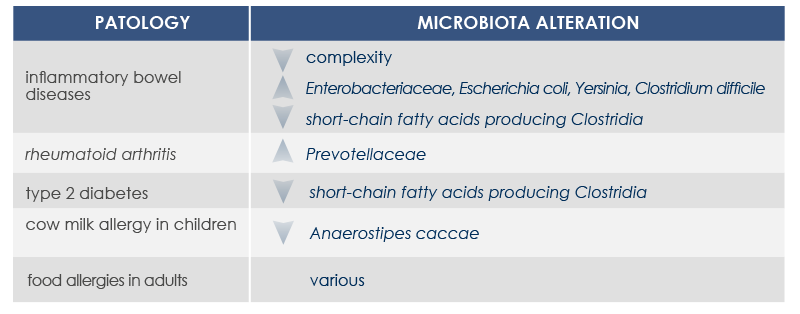
Microbiota changes can be associated with diseases characterized by the presence of inflammation. In fact, the loss of health-friendly bacteria reduces the ability to control the immune response. Moreover, inflammation can increase in response to the exaggerated proliferation of opportunistic bacteria characterized by strong invasiveness and inflammatory properties or able to proliferate in the presence of inflammation-associated metabolites.
Overuse of antibiotics, some eating habits, and chronic parasitic infection eradication can contribute to inflammation development too. In fact, they promote the growth of a microbiota lacking the resilience required to establish the balanced interaction between gut microbes and the organism that is needed to develop immune defenses and immune tolerance.
For example, mutations altering immune responses in the gut can induce the proliferation of bacteria promoting inflammation, and this proliferation is associated with inflammatory bowel diseases. Moreover, some microbiota features can contribute to the chronic inflammation state associated to the increased risk of cancer development and progression; for example, microbiota composition was associated with stomach, esophagus, colorectal, and breast cancer. Finally, microbiota is associated with inflammaging too.
GUT MICROBIOTA AND INFLAMMAGING
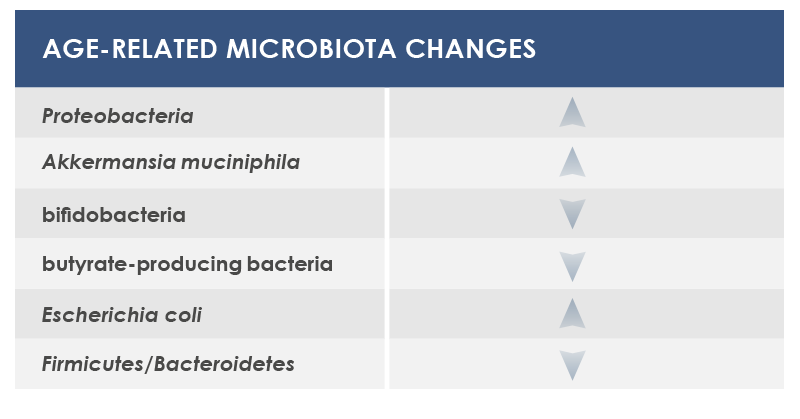
Gut microbiota is involved in both induction and maintenance of inflammaging. The mechanisms underlying this relationship include phenomena associated to the majority of age-associated diseases (enteric nervous system degeneration, gut motility alterations, decreased efficiency of the mucosal barrier) that are linked to increased proinflammatory cytokines and to changes in gut microbiota composition and stability.
In particular, during aging microbiota tends to lose the ability to ferment carbohydrates, to better ferment proteins, and to have a reduced diversity, with the increase of some bacteria species. External factors exert a little influence on some microbiota changes; that is why these shifts are considered as potential core features of elderly microbiota. Other changes are instead more dependent on lifestyle factors, for example on eating habits and drugs.
Among bacteria whose levels could change are included: Proteobacteria, that were associated to both local and systemic inflammation; Akkermansia muciniphila, that breaks down a gut barrier component, mucin; bifidobacteria, health-friendly lactic ferments; microbes producing butyrate, an inflammation regulator; and Escherichia coli.
In the blood, molecules from gut microbiota activate macrophages, generating a proinflammatory status that promotes atherosclerosis and that is associated with both cardiovascular diseases and vascular dementia. Moreover, cytokines and short-chain fatty acids variations associated with gut microbiota changes influence cognitive decline. Finally, the proinflammatory response stimulated by lipopolysaccharide promotes beta-amyloid protein production; this is an evidence of a possible link between gut microbiota and Alzheimer’s disease.
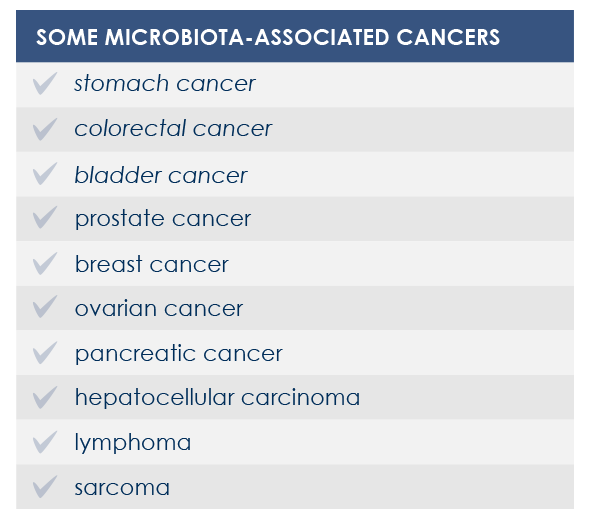
MICROBIOTA AND CANCER
The link between microbiota, inflammation and immune system influences cancer development both locally and at systemic level.
Factors playing a role in this association include innate and adoptive immunity, endocrine and nervous pathways, bacteria translocation, toxins or other molecules of bacterial origin, and modulation of systemic inflammation and oxidative stress.
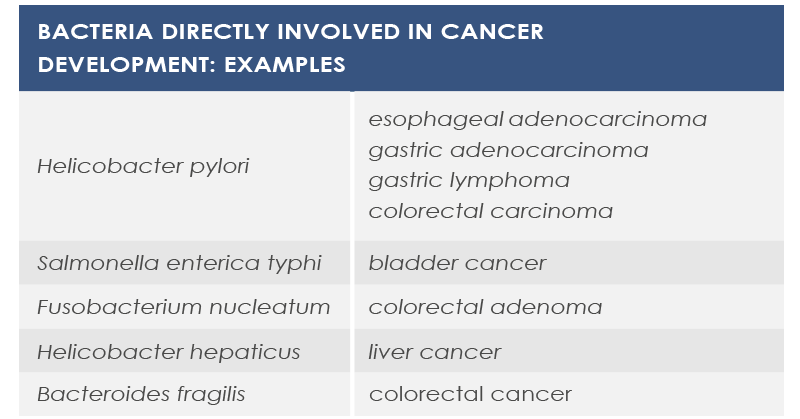
Sometimes cancer development depends on a single bacterial species that can modify signals and communication pathways, for example by means of toxins that modulate inflammation in tumor microenvironment, influence genomic stability of human cells, or epigenetically regulate host genes. Also diet can participate in this association. For example, some bacteria metabolizes meat-derived substances leading to the production of DNA damaging molecules.
In other cases cancer development depends on changes in microbiota composition or density. Moreover, several evidences point to the association between obesity – a well known cancer risk factor – and gut microbiota.
However, some gut bacteria exert a protective effect against cancer. Moreover, some prebiotics (substances promoting the growth and the activity of gut microbiota) can contribute to cancer prevention via their antioxidant properties and by reducing inflammation.
Finally, microbiota can be involved in anticancer therapies efficacy and adverse effects. For example, cyclophosphamide effect depends on gut flora, and also anticancer response to oxaliplatin, cisplatin and CpG oligonucleotides depends on gut microbiota. The microbiota of melanoma patients responding to anti-PD1 immunotherapy is characterized by greater diversity and abundance of Ruminococcaceae and Faecalibacterium, whereas Bacteroides species such as B. thetaiotaomicron and B. fragilis contribute to improving immune response against cancer induced by anti-CTLA-4 antibodies.
Other commensal bacteria can instead induce adverse effects, such as CPT-11 dose-limiting diarrhea.
GUT MICROBIOTA AND DIET
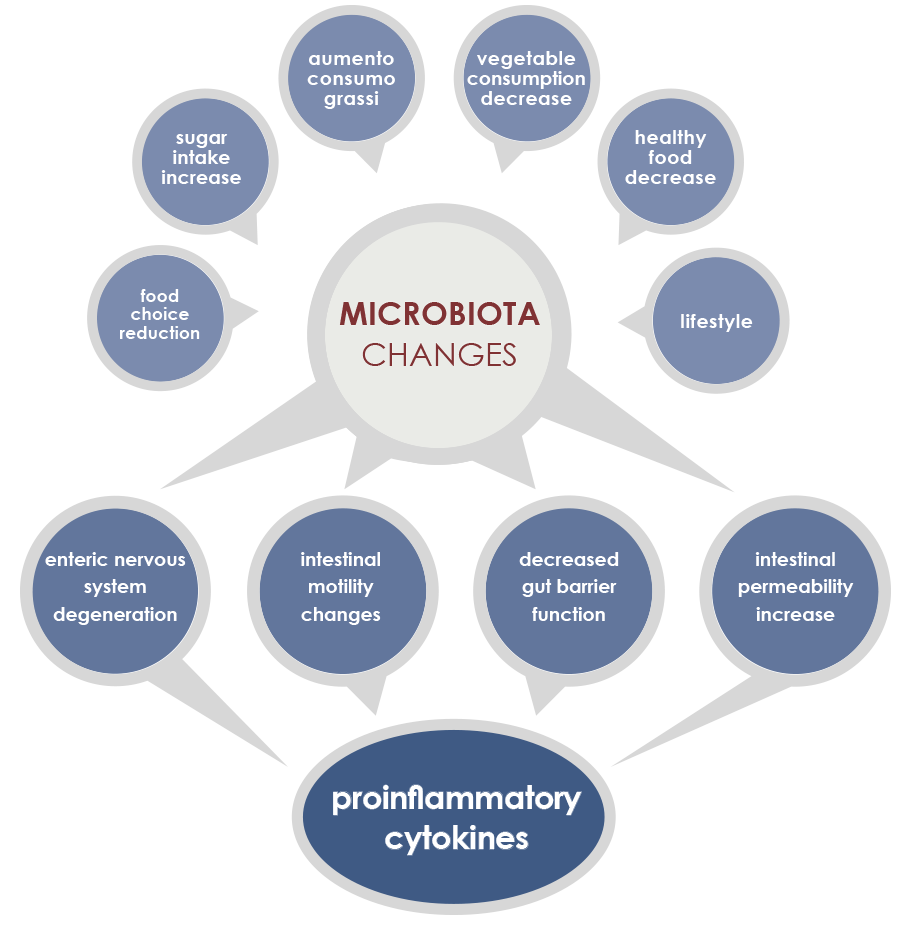
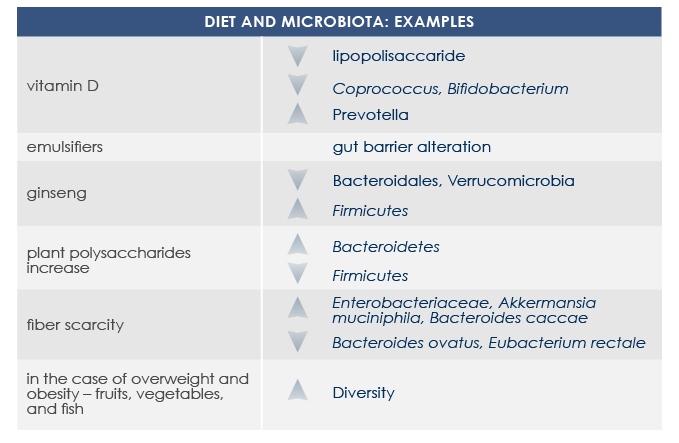
Diet is among the most important factors that modulates gut microbiota structure and functioning since very first days of life. Some nutrients (such as glycans, quinones, and flavonoids) directly interact with bacteria, promoting or inhibiting their growth. Moreover some food-derived substances indirectly influence gut microbiota via host metabolism and immune system. That is why microbiota responds to diet changes and prebiotics, probiotics, and symbiotics consumption is among current possible approaches to contrast metabolic problems associated with gut microbiota alterations.
Tailored diet-based approaches can promote gut microbiota changes in only one week.
Prebiotics benefits
Prebiotics are substances that increase bacteria growth or activity. Among most known are non-digestible fiber, which is present in a lot of fruit, vegetables and legumes.
Also phytoestrogens present in some berries act as prebiotics.

PROBIOTICS
Probiotics are live bacteria (lactobacilli and bifidobacteria) that, when ingested in the form of fermented foods or dietary supplements, are associated to health benefits. Probiotic-based dietary supplements are designed to replenish the gut with commensal bacteria associated with favorable metabolic properties. Multi-strain probiotics seem to be more efficient than single-strain probiotics.
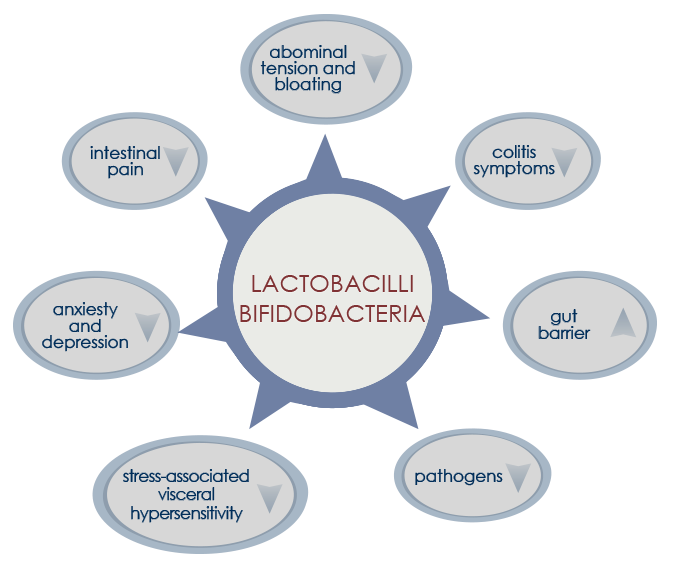
SYMBIOTICS
Symbiotics are products combining prebiotics properties to probiotics ones.
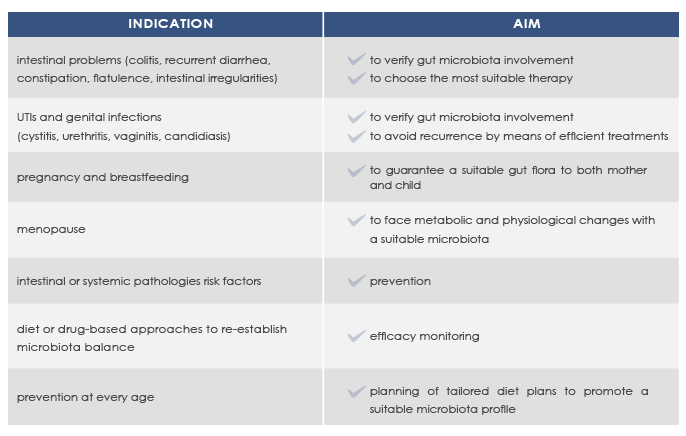
INDICATIONS FOR MICROBALANCE
Gut microbiota composition is unique for each individual, with some profiles associated with better health conditions.
Microbioma analysis can help promote a good health status and prevent or manage intestinal or systemic diseases such as colitis, recurrent diarrhea, obesity, and metabolic syndrome.
Next-Generation Sequencing-based microbioma analysis allows the analysis of both gut and vaginal microbioma without the obstacles that are characteristic of anaerobic microorganisms lab culture.
With MICROBALANCE, Bioscience Institute allows sampling at home.
Resulting profiles do not represent the diagnosis of a pathology, but allow qualified clinicians to elaborate diet plans to correct dysbiosis and to take into account a specific dietary supplements-based approach.