Indications for the G-Test
In what situations is the G-test recommended?
- Pregnancies in which invasive tests are recommended
- Pregnancies in which invasive testing is not intended
- Single or twin pregnancies
- Single pregnancies from assisted insemination (also from egg donation)
- Twin pregnancies from assisted insemination (also from egg donation)

Reliability of the test
Sensitivity and specificity are greater than 99%
- Sensitivity and specificity are greater than 99%
- Fully certified CE-IVD analysis protocol
- High resolution DNA sequencing (up to 60 million reads)
- Sensitivity is greater than 99.9% for trisomies 21, 18 and 13
- False positives for trisomies 21, 18 and 13 less than 0.1%
- Sensitivity is greater than 95.5% for whole genome screening
- Specificity is greater than 99.3% for any analysis option
- Detection of very small deletions/duplications
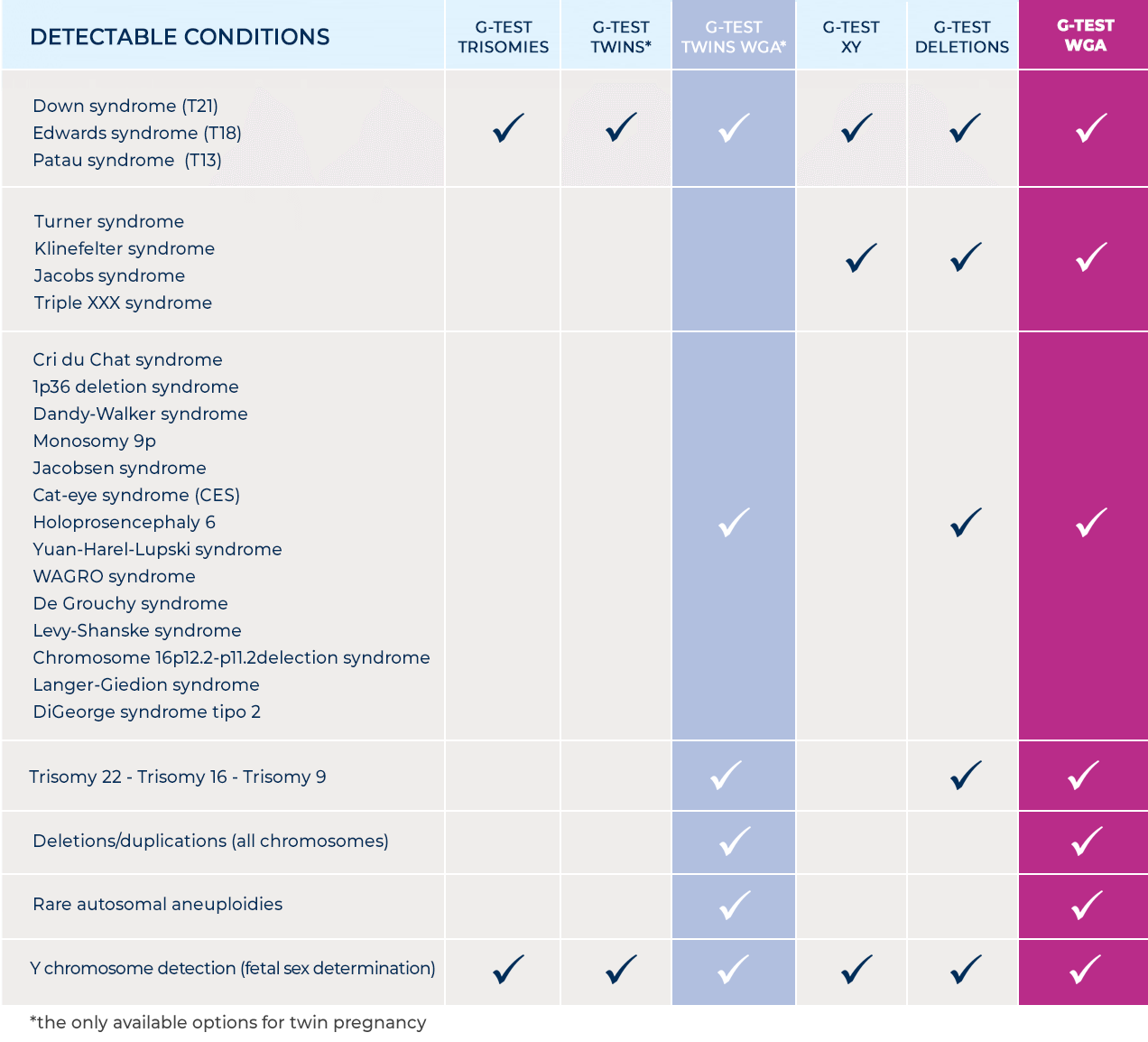
The G-Test WGA
The most thorough level of examination
The prenatal analysis of the whole genome (Whole Genome Analysis) is the most recent evolution of fetal DNA tests and the overcoming of obsolete and limited sequencing technologies on single chromosomes (still existing due to their low cost).
The WGA analysis makes it possible to broaden the search for aneuploidies and deletion/duplication syndromes to all chromosomes, detecting them even when they are very small.
How to undergo the G-Test
By contacting Bioscience Institute, through the online form at the end of the page or writing to info@bioinst.com, it is possible to receive all the preliminary information (timing, methods of execution, etc.) and indications on the nearest affiliated centers to contact to undergo the G-Test.
Withdrawal and transport
The maternal peripheral blood sample required for the test is approximately 8 ml; it can be performed starting from the 10th week of gestation using the CE-IVD certified test tube supplied in the collection and transport kit.
The blood sample is sent for analysis to the Bioscience Genomics laboratories, in an isothermal kit for the transport of category B biological material, in compliance with the UN3373 standard.
Why choose the G-Test?
The advantages of the prenatal test offered by Bioscience Genomics
- no risk to the health of the fetus or the mother
- sensitivity and specificity for trisomies 21,18 and 13 greater than 99.9%
- specificity is greater than 99.3%, for any analysis option
- automated and CE-IVD certified DNA extraction, sequencing and analysis protocol can detect very small anomalies (up to 7Mb) in all chromosomes, even in twin pregnancies
- high level of in-depth analysis for any analysis option (resolution of up to 60 million reads); in the event of an anomaly being detected, reimbursement is provided for any costs of in-depth diagnostic and/or genetic counseling (*)
- test results are available in approximately 5 working days
(*) There are some restrictions. For more information, you can contact the Bioscience Institute before undergoing the test.
Information on the test results
To date, more than 3 million G-Tests have been performed in over 52 countries.
Once it arrives at the Bioscience Genomics laboratories, the blood sample is taken over by the laboratory biologists and the results are ready in about 5 working days.
It should be noted that durations may vary: the analysis involves a series of rigorous quality controls to ensure the reliability of the result; among these is the verification of the quantity of fetal DNA present in the blood sample, which varies from one pregnant woman to another. In some cases, the low quantity of fetal DNA or the poor quality of the biological sample make it necessary to repeat the analysis and/or blood sampling.
If it is necessary to repeat the test due to the low concentration of fetal DNA in the maternal blood, the repeat will be free of charge.
Request G-Test
The safe, simple, fast, validated prenatal test
Contact Bioscience Institute to find the Center closest to you where you may book the G-Test®, or fill out the request form to be contacted without obligation by one of our trusted biologists.
(*) Required fields