Follow Bioscience Institute

Solid cancer
Cancer is a genetic disease. The accumulation of somatic mutations in the DNA of a cell leads to deregulation of its physiological functions resulting in uncontrolled growth eventually leading to cancer development. Once established, tumors shed DNA that can reach the circulatory system (circulating-free DNA or cfDNA).
Thus, monitoring the emergence of somatic mutations present in cfDNA may allow the detection of cancer at very early stages.
Early stage tumors with no metastatic spread have a significant better chance to be successfully treated. Based on the analysis of cfDNA using Next Generation Sequencing technology (NGS), HELIXAFE, performed on a regular base, provides a mutation frequency monitoring system and a risk assessment for tumor incidence. In the last decade, the NGS technology has revolutionized medical genetics and pathology allowing to inexpensively sequence the human genome at high speed and accuracy.
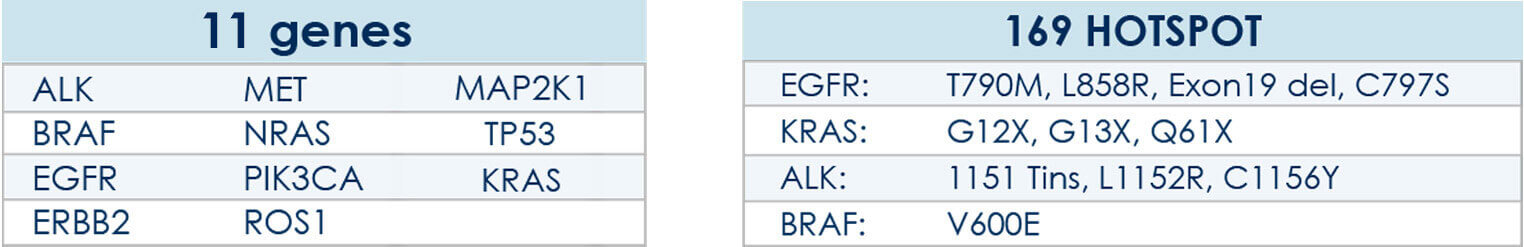
Using a proprietary algorithm developed at the Bioscience Genomics, HELIXAFE annual prevention program allows the monitoring of mutations frequency via the interrogation of 2800 hotspots of mutations over 50 solid tumors-associated genes.
OBJECTIVE ASSESSMENT RISK
Currently the risk assessment of solid cancers is based on the evaluation of the clinical medical history and familiarity of a patient, along with the analysis of certain biomarkers (e.g. PSA for prostate tumor) at a specific time point. In many cases however, this approach fails to detect cancer at its early onset.
Using a monitoring approach, HELIXAFE analyzes in real-time the emergence of cancer-associated mutations through NGS technology generating a risk score via customized proprietary software.
NON-INVASIVE
cfDNA can be easily obtained from just a 10 ml sample of full blood. HELIXAFE annual prevention program enables the detection of mutations associated with cancer risk development in real-time, or to follow-up mutations already detected in previous analyses, thus informing on the evolution of genetic aberrations. For patients at high-risk as defined using HELIXAFE, cancer-associated genetic alterations will be measured using SCED (Solid Cancer Early Detection). SCED ensures an accurate mutational analysis of cfDNA (at allelic frequency as low as 0.05%) with the aim of early cancer onset detection at stage were chances to be cured are high.

Lung Cancer
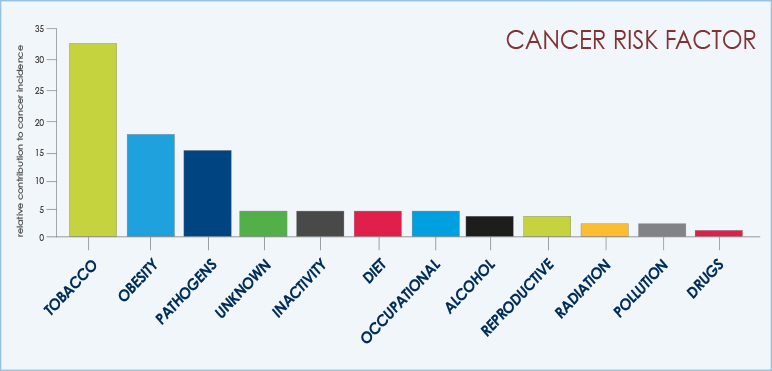
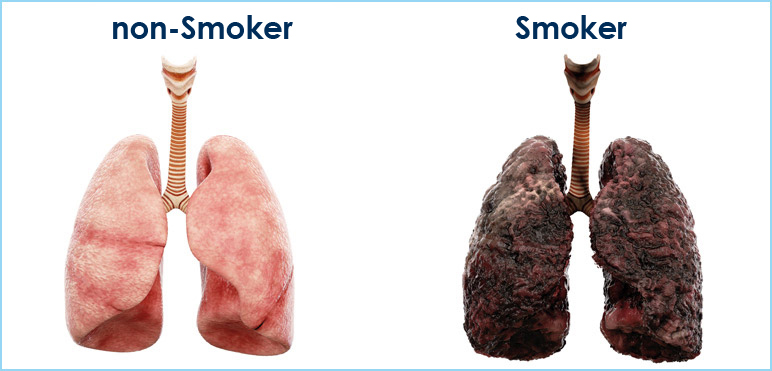
The main cause of lung cancer is cigarette smoke. Several studies indicate that there is a clear dose-effect relationship between inhaling tobacco and development of lung cancer.
Studies report that the risk of having lung cancer is 14 times higher among smokers than non-smokers (up to 20 times for heavy smokers, e.g. 20 cigarettes per day).
Cigarette smoke is responsible for the vest majority of lung tumors.
Air pollution, family history of lung cancer, as well as various pathological conditions affecting the normal lung physiology may also contribute to increase the possibility of being affected by this disease.

THE CONTINUOUS MONITORING OF A GENETIC MUTATIONAL PROFILE
Smokers, or people at risk of developing lung cancer, can undergo a yearly checkup of their mutational profile to monitor the potential onset of lung cancer.

Bowel cancer
HELIXCOLON is a prevention program that provides yearly assessment of 14 genes and 246 hotspots associated to the development of colorectal cancer.
Currently, screening tests used to detect colorectal cancer are based on blood detection in fecal samples, or in some cases the rectum sigmoidoscopy.
In western countries, colon-rectum cancer represents the second most common malignant tumor and it has a mortality rate of 13% at 5-years after diagnosis.
The occurrence of colorectal cancer is quite rare before the age of 40y and it is increasingly frequent from the age of 60y reaching a peak at age 80y, affecting men and women equally.

Risk factors concerning colorectal cancer development obesity, genetic predisposition, age, inflammatory chronic bowel diseases and clinical history of colon polyps play a major role.
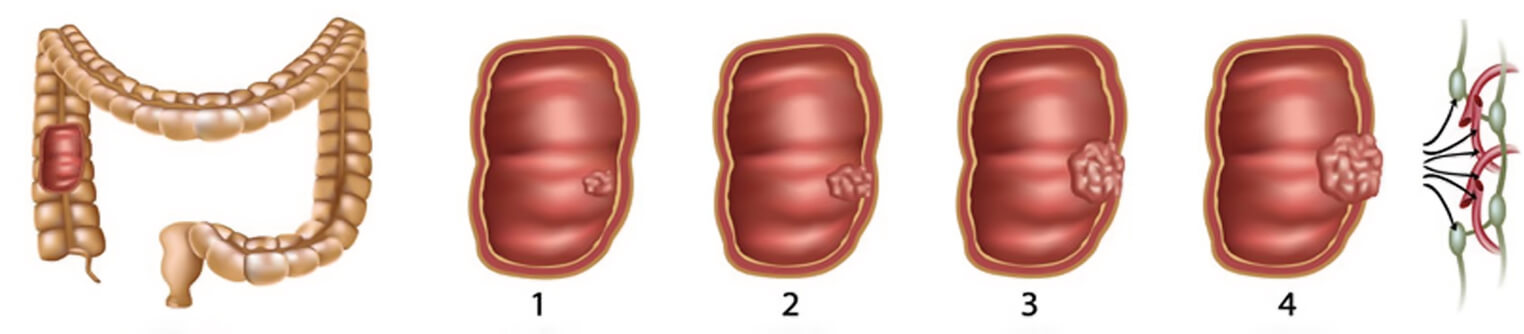
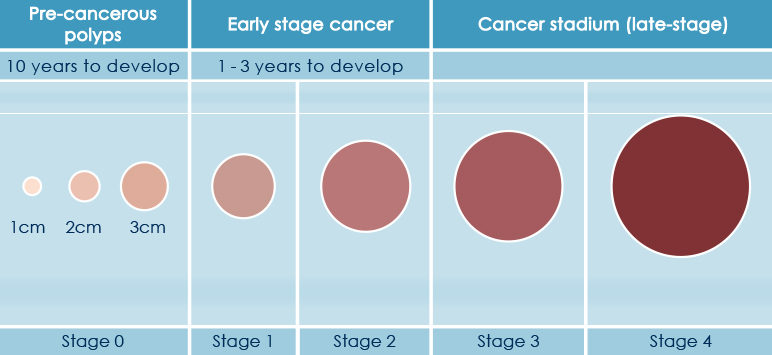
Colorectal cancer is often silent, having mild clinical symptoms till late stages and is often resulting from the evolution of a benign lesion (as the adenomatous polyps) acquiring genetic abnormalities over relative long period of time (usually several years to decades).
Thus, HELIXCOLON prevention program would ensure a timely identification of degenerating lesions allowing a prompt intervention strategy.

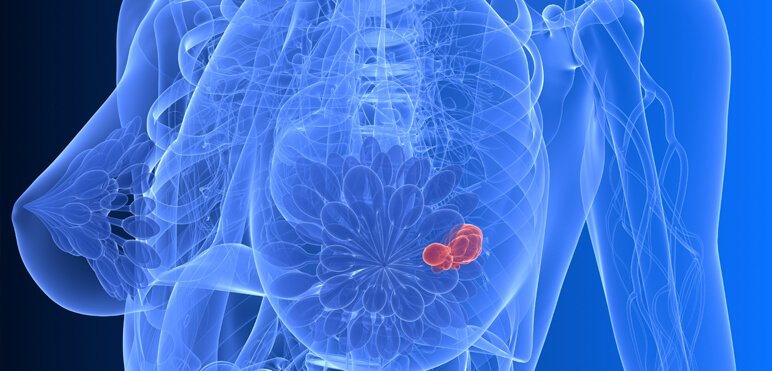
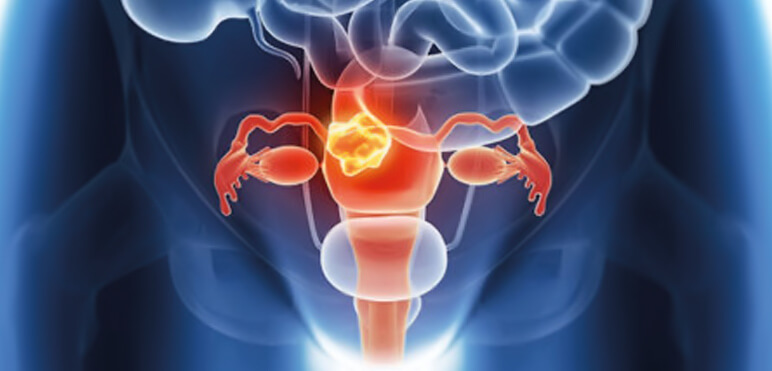
Breast and Ovarian Cancer
Breast and ovarian cancer are diagnosed to almost two million women each year worldwide. Risk factors include genetic predisposition, late pregnancy, a history of hormone replacement therapy, contraception or ovarian stimulation.
HELIXGYN is a yearly prevention program for women at risk of developing breast or ovarian cancer.
HELIXGYN monitors the occurrence of specific mutations that arise in the context of breast and ovarian cancer.
TOS the relationship between the hormone replacement therapy during menopause and the risk to develop several tumors has been a debated subject for decades. Hormone replacement therapy taken after menopause increases the risk to develop breast cancer as a function of treatment duration. The risk of endometrial hyperplasia, which could be a precursor for endometrial cancer, has been shown to increase in cases where only estrogens are administered. HELIXGYN is also designed to assess the safety of hormone replacement therapy.
FIVET growing scientific evidence suggest that some sensitive tissues such as those of breast, uterus, cervix and ovary, may be subject to tumor formation after prolonged hormone stimulation.Studies have underlined a link between cancer development and treatment against infertility. Also in this case, hormones can accelerate the growth of tumor cells that may be already present in some tissues. HELIXGYN can monitor the occurence of cancer-associated mutations that may arise as a consequence to prolonged hormone stimulation.
CONTRACEPTION Hormonal therapy used as contraceptive contains endocrine hormones, which may stimulate the growth of tumors cells. HELIXGYN can help to assess the safety of contraceptive hormone treatment or hormone replacement therapy by monitoring the emergence of mutations that are associated to breast and ovarian cancer development.