
These are diseases whose appearance is due to an error at the time of meiosis, to a chromosomal aberration or to a variant that appears for the first time (“de novo”) in a family member.
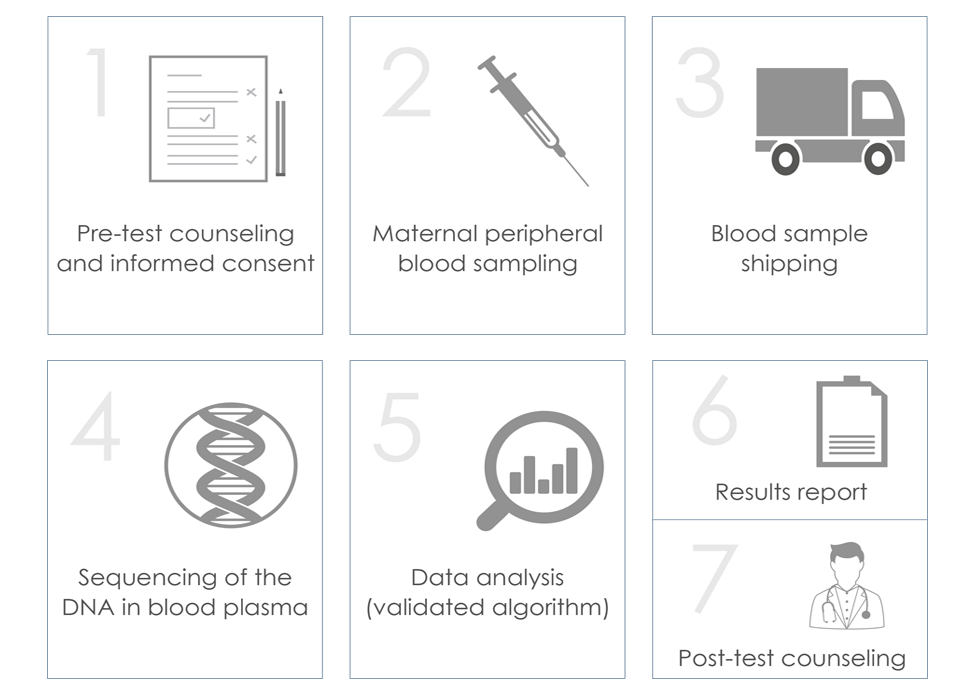
OMNIPT is performed on a maternal peripheral blood sample taken after the 10th week of gestation (within the 24th). In a few days, the DNA is extracted from the plasma, sequenced and analyzed using sophisticated bioinformatic calculation algorithms.
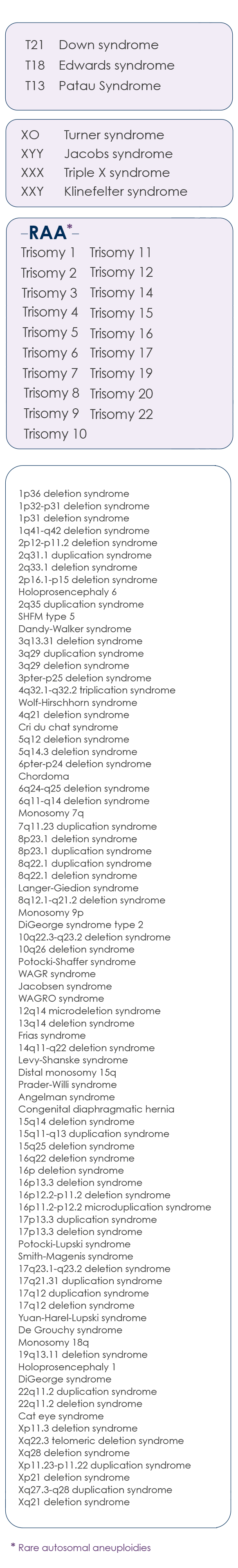
Chromosomal anomalies analyzed with the OMNIPT test

It has been observed that the incidence of DE NOVO MUTATIONS * rises with increasing parental age, especially when this is attributed to the father.
(*) MUTATIONS DE NOVO
The mother or father has a mutation in a single germ cell and passes it on to the child, or a mutation occurs in the zygote (fertilized egg) during the very first cell divisions.
Chromosomal anomalies analyzed with the OMNIPT test
It has been observed that the incidence of DE NOVO MUTATIONS * rises with increasing parental age, especially when this is attributed to the father.
(*) MUTATIONS DE NOVO
The mother or father has a mutation in a single germ cell and passes it on to the child, or a mutation occurs in the zygote (fertilized egg) during the very first cell divisions.

Indications
The OMNIPT test is indicated in case of:
- Single pregnancies in which the age of the parents is a risk factor
- Single pregnancies in which invasive testing is not recommended
- Single pregnancies in which invasive prenatal diagnosis is indicated
- Single pregnancies from assisted insemination (homologous or heterologous)
Advantages
- It is based on a simple maternal blood sample
- It is performed after the 10th week of gestation (within the 24th)
- It can detect 110 chromosomal diseases with relative clinical interpretation
- It can detect 27 monogenic diseases with relative clinical interpretation
- Provides compensation in the event of failure to detect (*)
- It provides for a reimbursement of expenses (*) for any further diagnostic and/or genetic counseling
Reliability
- The only test in the world capable of detecting 110 chromosomal and 27 monogenic diseases with relative clinical interpretation
- A clinical study of over 146,958 women makes it by far the most validated fetal DNA test for trisomies
- Sensitivity greater than 99% for trisomies 21, 18 and 13
- Sensitivity greater than 90% for the detection of deletions and duplications, even submicroscopic (up to 3Mb)
- Sensitivity and specificity greater than 99% for monogenic diseases
Quality assurance
The guarantee of reliability, coming from the widest case history in the world (more than 5 million tests performed), has made it possible for each pregnant woman who requests it, access to a free insurance policy *. In case of failure to detect some aneuploidies, it is possible to receive compensation or, in the case of in-depth diagnostic and/or genetic counseling, a reimbursement of expenses.
* There are some restrictions. For more information, you can contact the Bioscience Institute before undergoing the test.
Procedure
The maternal peripheral blood sample required for the ULTRANIPT DG test is approximately 8 ml. The ULTRANIPT DG test can be performed starting from the 10th week of gestation (within the 24th) using the CE-IVD certified test tube provided in the collection and transport kit. The blood taken is transported in an isothermal kit for category B biological material, in compliance with the UN3373 standard.
Results management
Results for chromosomal diseases are available in approximately 8 business days. It is important to point out that waiting periods may vary: the analysis involves a series of rigorous quality controls aimed at guaranteeing the reliability of the result; among these is the verification of the quantity of fetal DNA present in the blood sample, which varies from one pregnant woman to the other. In some cases, due to the low quantity of fetal DNA, it is necessary to repeat the analysis and/or blood sampling. If it is necessary to repeat the test due to the low concentration of fetal DNA in the maternal blood, the repeat will be free of charge.
Request OMNIPT
The prenatal test with a more extensive and targeted level of screening
Contact Bioscience Institute to find the Center closest to you where you may book the OMNIPT Test, or fill out the request form to be contacted without obligation by one of our trusted biologists.
(*) Required fields
References
• Zhang H, et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146.958 pregnancies. Ultrasound Obstet Gynecol.2015 Jan 19.
• Chen S, et al. A method for noninvasive detection of large fetal deletions/duplications by low coverage massively parallel sequencing. Prenat Diagn.2013
• Liu et al. Performance evaluation of NIPT in detection of chromosomal copy number variants using low coverage whole genome sequencing of plasma DNA. Plos One, 2016
• Pan X, et al. Noninvasive fetal sex determination by maternal plasma sequencing and application X-linked disorder counseling. J.Matern Fetal Neonatal Med. 2014 Dec.
• Jiang et al. Noninvasive fetal Trisomy test: an advanced noninvasive prenatal diagnosis methodology for fetal and sex chromosomal aneuploidies. BMC Medical Genomics, 2012.


